
The organizations going for ISO 17025 accreditation are always in search of readymade documentation to save time. Global Manager Group has globally reputed experienced team of consultants having rich experience in quality management system for laboratories and test laboratory accreditation. Our consulting team has prepared ISO 17025 documents and globally many laboratories from USA, UK, Saudi Arabia, Qatar, Oman, UAE, Kuwait Middle East, Europe, Asia are already used our documents and ready to use templates during ISO 17025 accreditation and more than 70 laboratories are already accredited. The document toolkit is designed to address a wide range of quality related issues including and take care of all complex issues for any type of testing done in the testing laboratories. The editable documents are addressing the requirements for establishing the best laboratory system with good laboratory practices and are prepared based on the rich experience of our consultant for calibration and test laboratory accreditation. Information on Test laboratory accreditation - ISO 17025 documents with the complete list of more than 100 editable files are provided in demo. The user can easily modify the name of the test laboratory, its logo and other things required to your test laboratory for the preparation of accreditation iso 17025 documents of your test laboratory The ISO 17025:2017 accreditation audit is done by accredited certifying body auditors.


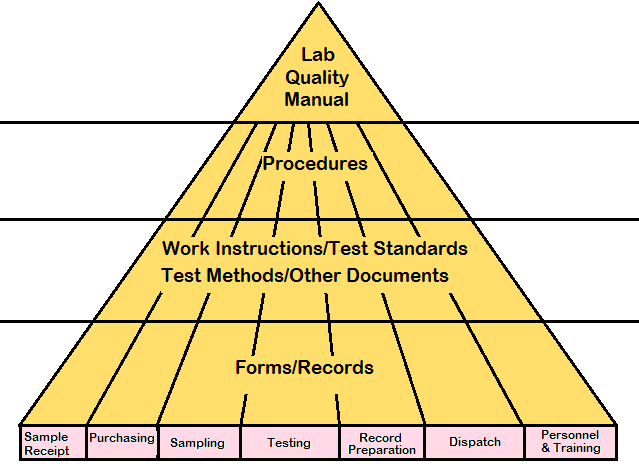
Formats and Templates (70 formats): A set of 70 templates and forms give a total idea to establish the system for the laboratory.Exhibits (8 exhibits): The 8 exhibits including an exhibit for calibration periodicity of instruments are given.ISO 17025 Procedures (21 procedures): It includes 21 procedures to implement the system in the test laboratory and comply with accreditation standard requirements covering mandatory requirements of documented procedures.
#Iso 17025 2017 quality mannual template manual#
ISO 17025 Manual (8 chapters): A sample laboratory quality system - iso 17025 manual with the quality policy and each chapter is explaining in plain English macro level management strategy and commitment and how the laboratory system is implemented.The contents of the test laboratory document kit include more than 100 document files as listed below: Our documents are editable and many organizations and ISO 17025 consultants are using our documents.


 0 kommentar(er)
0 kommentar(er)
